An explanation and the latest news on COVID-19 Vaccines
As of November 1st, the United States has averaged 80,755 new COVID-19 cases per day, a 43 % increase from the average two weeks earlier (1). According to the New York Times database, more than 9,209,500 people in the US have been infected with the coronavirus and at least 230,500 have died (1). There are only two ways to end a pandemic: a gradual decrease in infection and death rate or a vaccine. While the former has not been successful given the recent data, companies around the world are racing to find the latter, a vaccine for COVID-19.
Vaccines work by training the immune system to recognize and combat targeted viruses and bacteria. If the body is exposed to these viral or bacterial germs later, the body will immediately destroy them, thus preventing infection. The overall production of vaccines has five major stages: Preclinical Testing, Phase I, Phase II, Phase III, and approval and release (3). Before Phase I starts, companies identify and develop vaccine candidates and test them first on animals; if successful, the FDA approves this vaccine candidate to move on to the human clinical trials (3). In Phase I, the vaccine is tested on a group of less than 100 healthy adults, and the adults are monitored for their safety and the immune response that the vaccine generates (3). Phase I also allows scientists to determine the most effective dose and expand the safety of the vaccine (4). Once the vaccine is approved for Phase 2, a group of hundreds of people is tested. If there are no major complications and side effects, the clinical trial will proceed to Phase 3. For COVID-19 specifically, Janssen Pharmaceuticals, a pharmaceutical company headquartered in Belgium that researches a wide range of human medical disorders, plans to vaccinate tens of thousands of healthy people (4). In this stage of the clinical trial, the subjects will be randomly assigned to a group; one group will receive the vaccine while the other group will receive a placebo (4). “It may be that some people do go on to develop COVID-19, even after having been vaccinated, but they may have substantially milder symptoms than those who develop COVID-19 in a control group,” says Macaya Douoguih, M.D., MPH, Head of Clinical Development & Medical Affairs, Janssen Vaccines (4).
After Phase 3, regulatory bodies such as the European Commission or the FDA review the clinical data to ensure that the vaccine is safe and effective (3). Once the vaccine has been approved, it will be produced and distributed to the public (3). Although vaccines typically require years of research and testing before being released to the public, scientists are racing to produce a safe and effective coronavirus vaccine by the end of 2020. While vaccines for diseases such as Influenza and Yellow Fever took decades for scientists to develop, many coronavirus vaccine trials are already in the final stages of the testing process.
As of October 16, there are more than 100 vaccine candidates under development, with 41 already in clinical trials on humans (5). The COVID-19 vaccine clinical trials began in January with the intensive research of the coronavirus genome. In March, the first vaccine trials in humans began; companies such as Moderna, BioNTech, and Johnson & Johnson were among the first to reach Phase 3 of the clinical trials (5). However, a few trials have been recently paused due to unexpected complications. In September, AstraZeneca’s vaccine trial was paused after two volunteers became seriously ill. A month later, on October 12, Johnson and Johnson announced that they were pausing its vaccine trial due to an “unexplained illness” in a trial patient (6). The company and the Data Safety Monitoring Board will review the participant’s case before resuming the trial. On the following day, Eli Lilly also suspended their trials due to an aggregate health discrepancy between the experimental group and the control group; the Data Safety Monitoring Board found that the patients who had received the vaccine showed an undisclosed difference in their “clinical status” than those who had received a placebo (7) Although it is disappointing that these large-scale COVID-19 vaccine clinical trials are paused, scientists are sticking to their protocols to avoid any unforeseen side effects when the vaccine is distributed to the public.
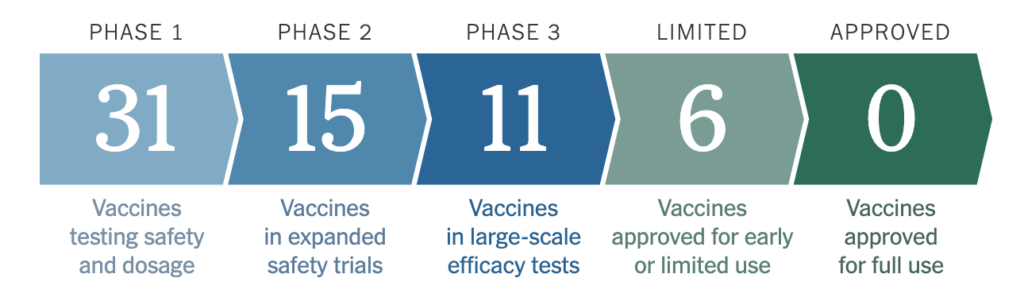
An overview of the number of COVID-19 vaccine clinical trials in the US in different stages.
Amidst the upcoming presidential election, President Donald Trump has made ambitious claims on the approval date and the distribution of the coronavirus vaccine. “We’ll have manufactured at least 100 million vaccine doses before the end of the year,” Trump said at a White House news conference (11). “We expect to have enough vaccines for every American by April” (11). President Trump’s expectations, however, conflict with the scientific predictions for the development and the distribution of the coronavirus vaccine.
Pfizer, one of the front-runners in developing a COVID-19 vaccine, says results will not be ready until mid-November at the earliest (9). The FDA announced earlier in October that before it reviews a coronavirus vaccine application, a company must have safety data that extends for at least two months (9). Unless the FDA is willing to approve the Pfizer trial or any Phase 3 trial immediately, there is a minimal chance that a vaccine will be ready by early November. Even if a vaccine can be approved and licensed in late 2020 or during 2021, there are still many unknowns concerning its potency, safety, and availability. According to the World Health Organization, healthy young people might not receive the coronavirus vaccine until 2022 as public health officials will focus on immunizing the elderly and most vulnerable groups first (10). As the coronavirus is changing constantly, it is unclear how long the vaccine will be effective for, or if a re-dosage of the drug will still be as effective. The current state of COVID-19 vaccines is changing rapidly every day; some vaccine trials are moving forward while others are being paused. Neither the FDA, WHO, nor President Trump can predict the exact date when a vaccine will be ready for the public. As for right now, we will have to continue to prevent the coronavirus by social distancing until that day comes.
– Abby Chen
References
- The New York Times. (2020, March 03). Covid in the U.S.: Latest Map and Case Count. Retrieved from https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html
- The push for a COVID-19 vaccine. (n.d.). Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines
- The Clinical Trial Process for a COVID-19 Vaccine. (2020, March 13). Retrieved from https://deep6.ai/the-clinical-trial-process-for-a-covid-19-vaccine/
- Levine, H. (2020, October 16). The 5 Stages of COVID-19 Vaccine Development: What You Need to Know About How a Clinical Trial Works. Retrieved from https://www.jnj.com/innovation/the-5-stages-of-covid-19-vaccine-development-what-you-need-to-know-about-how-a-clinical-trial-works
- Corum, J., Wee, S., & Zimmer, C. (2020, June 10). Coronavirus Vaccine Tracker. Retrieved from https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- Weintraub, K., & Weise, E. (2020, October 14). Eli Lilly and Johnson & Johnson have paused COVID-19 vaccine trials. Why experts say that’s reassuring, not frightening. Retrieved from https://www.usatoday.com/story/news/health/2020/10/13/covid-19-vaccine-trials-on-hold-eli-lilly-johnson-johnson/3643936001/
- Zimmer, C. (2020, October 14). 3 Covid-19 Trials Have Been Paused for Safety. That’s a Good Thing. Retrieved from https://www.nytimes.com/2020/10/14/health/covid-clinical-trials.html
- Fourth large-scale COVID-19 vaccine trial begins in the United States. (2020, September 23). Retrieved from https://www.nih.gov/news-events/news-releases/fourth-large-scale-covid-19-vaccine-trial-begins-united-states
- Harris, R. (2020, October 16). Pfizer COVID-19 Vaccine Won’t Be Ready By Election Day. Retrieved from https://www.npr.org/sections/coronavirus-live-updates/2020/10/16/924502362/pfizer-covid-19-vaccine-wont-be-ready-by-election-day
- Feuer, W. (2020, October 14). Healthy young people might not be able to get the coronavirus vaccine until 2022, WHO says. Retrieved from https://www.cnbc.com/2020/10/14/coronavirus-healthy-young-people-might-not-be-able-to-get-the-vaccine-until-2022-who-says.html
- Fabian, J., & Wingrove, J. (2020, September 18). Trump Digs In on Vaccine Vows That Risk Letdown or Wide Mistrust. Retrieved November 01, 2020, from https://www.bloomberg.com/news/articles/2020-09-18/trump-s-promise-of-october-vaccine-risks-letdown-or-rejection
Images
- https://www.aljazeera.com/wp-content/uploads/2020/10/2020-09-02T212304Z_1121128429_RC2LQI9ELBRO_RTRMADP_3_HEALTH-CORONAVIRUS-VACCINES-COVAX.jpg?resize=770%2C513
- https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html

