Current approaches and future developments of organ preservation technology
During kidney transplantation, from the moment the kidney is harvested from its donor, it may take hours or even days to reach its recipient. Proper preservation of the organ during this time is crucial, as kidneys are highly susceptible to ischemic and hypoxic damage, both of which impair blood flow and cause kidney failure. To avoid surgical complications and optimize the chances of organ function, using the best organ preservation methods is vital in having a successful transplant (1).
Static cold storage (SCS) is the traditional method of organ preservation. It has been the primary preservation method for the past 50 years and is still widely used today. The first cold storage solutions in clinical transplantation were introduced in 1969 and continued to advance throughout the decade. A solution developed by Folkert Belzer and James Southard from the University of Wisconsin in the 1980s is the solution primarily used in kidney transplants today. The kidney is placed in a hypothermic solution, usually around 40 degrees Fahrenheit, to stop the organ’s metabolism and enzymatic processes. By freezing the kidney’s natural functions, SCS prevents ATP depletion, a process in which cells naturally degrade as they would if placed outside of the body under normal temperatures. The hypothermic solution is composed of impairments, colloids, buffers, electrolytes, and antioxidants. Each element serves a different purpose, including minimizing cellular swelling (2).
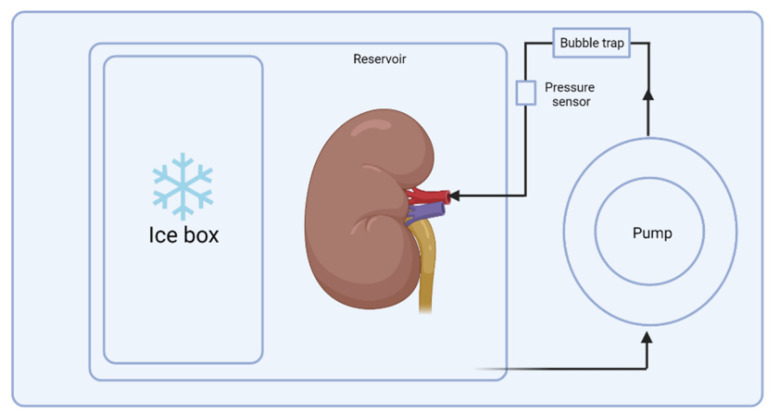
A newer alternative method of organ preservation is hypothermic machine perfusion, which provides additional support to the preservation process, as it allows doctors to closely monitor kidney function. A machine continuously pumps a preservation solution through the kidney. Hypothermic machine perfusion is shown to improve rates of graft survival in kidney transplants, which is a sign of a successful transplant. As the need for more viable kidneys increased in the 1990s, efforts were made to further develop perfusion machines. Compared to SCS, the perfusion technique is expensive and requires additional complex machinery (3). However, by 2015, approximately 25% to 35% of all transplanted kidneys in the United States used machine perfusion (4).
A study published in the New England Journal of Medicine followed 672 kidney transplant patients. Half of the surgeries used static cold storage while the other half used hypothermic machine perfusion. The study showed that the use of hypothermic machine perfusion significantly reduced delayed graft function, providing more successful transplants (5). Given this data, as medicine and machinery continue to evolve, it is likely that we will see a shift to hypothermic machine perfusion as the main technique of organ preservation.
Bibliography
- Chouchani, E. T., (2016, Nov 5). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. Retrieved from https://doi.org/10.1038/nature13909.
- Rijkse, Elsaline. (2020, Jan 18). Machine perfusion in abdominal organ transplantation: Current use in the Netherlands. World Journal of Transplantation. Retrieved from https://doi.org/10.5500/wjt.v10.i1.15.
- (2023). Exploring the benefits of machine perfusion in organ transplantation. University of Cincinnati Health. Retrieved from https://www.uchealth.com/en/media-room/articles/exploring-the-benefits-of-machine-perfusion-in-organ-transplantation#:~:text=Machine%20perfusion%20is%20a%20technique,supply%20and%20longer%20preservation%20time.
- N, Goulamhoussen (2022, Jan 1) Factors Associated With the Use of Hypothermic Machine Perfusion in Kidney Transplant Recipients: A Multicenter Retrospective Cohort Study. National Library of Medicine. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9434662/.
- C, Moers (2009). Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. New England Journal of Medicine. Retrieved from https://doi.org/10.1056/nejmoa0802289.
Images