How a specific antibody in bulk fights the COVID-19 virus
What causes your body to spring into action? When you’re not feeling well, your body stimulates an immune reaction. This action causes white blood cells (WBCs) to produce Y-shaped proteins called antibodies; their goal is to stop the cycle of foreign, harmful substances (pathogens) from entering our cells (10). Antibodies perform their job with B-cells and T-cells. B-cells are in WBCs and aid in creating antibodies that recognize the disease, while T-cells kill infected cells in the body. Antibodies aim to prevent antigens, molecules that stimulate an immune response, from entering the body by binding to its surface or the epitope (6). An antibody blocks the antigen’s epitope — its pathway into the cell.
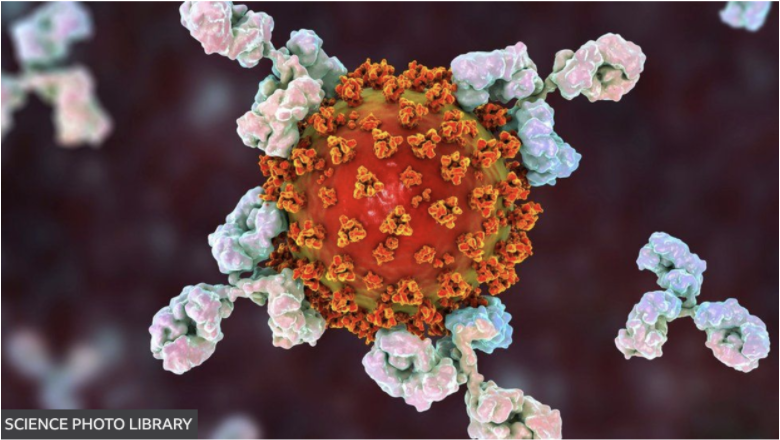
Specific antibodies bond to the epitope depending on which antigen the body is fighting. Often, various antibodies bind to antigens in order to return to homeostasis rapidly, creating a polyclonal antibody reaction.
Now, when the body experiences a myriad of symptoms because of one disease, laboratory discoveries state that monoclonal antibodies (mAbs) could aid in the recovery. “We are at the beginning of a new era of immunochemistry,” said César Milstein, a biochemist who worked alongside Georges Köhler and Niels Ka Jerne (2). In the 1990s, the trio discovered that isolating a single antibody and mass-producing it could supplement human antibodies to combat disease. The scientists focused on cancer research and believed that by allowing mAbs to fight cancer, the patient’s immune system would “refocus” and block the pathogen. Their experiment consisted of extracting B-cells from the lymph nodes of an infected mouse and attaching them to a growing myeloma, a tumorous cell. This combination caused the formation of a hybridoma, which is an antibody-producing factory that can be traced back to a parent WBC. The scientists concluded that this solution of B-cells and hybridomas would maintain an antibody-producing factory specific to cancer. Subsequently, the next generations of antibodies would not kill additional cells (3, 7).

Scientists are continuously testing a mAbs treatment for cancer, which has been proven to slow its growth throughout the body. The COVID-19 pandemic sparked a new application for mAbs. As coronavirus continues to overwhelm the world, it is vital for researchers to create new treatments in hospitals. The coronavirus is defined by one of its four major structural proteins — its spikes on each genome, which helps the disease enter more cells in a quicker amount of time for a prolonged time period.
Anti-SARS-CoV-2 mAbs, which consist of essentially a single antibody in bulk, targets the spike protein in the antigen (1). The blocking hinders the virus’ entrance into other cell membranes, where its genome would have otherwise been released for replication. The antibody neutralizes the effect of the pathogen by changing the genome composition so that it will not continue to infiltrate cells. Additionally, the consistent blocking of the antigen pushes the immune system to act in a more complete response, which is beneficial when caring for high-risk patients (5).
The hope in medicine is that mAbs treatments will withstand everchanging mutations of SARS-CoV-2. Because of unpredictable changes in the virus’ spike proteins, certain antibody treatments do not target the Omicron variant (4). However, some companies such as GSK-Vir Biotechnology have confirmed that their antibody treatments are protective against all known 37 mutations of the virus (9). As we continue to navigate the constant changes of the pandemic, monoclonal antibodies seem promising in their efforts of reducing the viral load, yet come with challenges of targeting all possible mutations.
Bibliography
- Anti-SARS-CoV-2-Monoclonal Antibodies/COVID-19 Treatment Guidelines. (2021). COVID-19 Treatment Guidelines; COVID-19 Treatment Guidelines. Retrieved from https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
- Breakthrough: Dr. César Milstein And The MAbs. (2019, July 19). NFCR. Retrieved from https://www.nfcr.org/blog/cesar-milstein-mabs-breakthrough/
- Ferguson, J.P.D. (2019). Monoclonal antibodies. Salem Press Encyclopedia of Health.
- Hospitals Scramble as Antibody Treatments Fail Against Omicron. (2022). The New York Times. Retrieved from https://www.nytimes.com/2021/12/21/health/covid-monoclonal-antibodies-omicron.html
- How do antibodies neutralize the novel coronavirus?| Scripps Research. (2022) Scripps.edu. Retrieved fromhttps://www.scripps.edu/covid-19/science-simplified/ antibodies-neutralize-the-novel-coronavirus/
- Khan Academy. (2022). Khanacademy.org. Retrieved from https://www.khanacademy.org/ science-in-in-class-12-biology-india/xc09ed98f7a9e671b-in-in-human-health-and-disease/xc09ed98f7a9e6716:in:in-types-of-immunity-and-the-inmune-system/a/hs-the/ immune-system-review.
- Monoclonal antibody drugs for cancer: How they work. (2021). Mayo Clinic. Retrieved from https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/ monoclonal-antibody/art-20047808.
- Morelle, R. (2020, September 14). Coronavirus: Monoclonal antibodies to begin UK trial. BBC News; BBC News. Retrieved from https://www.bbc.com/news/health-54120753
- Third COVID-19 antibody is authorized. (2021). Chemical & Engineering News; American Chemical Society. Retrieved from https://cen.acs.org/pharmaceuticals/biologics/ Vir-GSK-monoclonal-antibody-authorized/99/i21
- Vanderpuye, O.A., PhD. (2020). Antibodies. Retrieved from Salem Press Encyclopedia of Health.
