Insights From Dr. Ott About His Revolutionary Research to End Transplant Waitlists
Right now, if your kidney fails, your next few years of life may be completely altered. At a serious level, you will need kidney dialysis, a treatment that filters extra blood from the body while you are placed on the 3-5 year transplant waitlist (1). Quality of life on dialysis is very poor. You will frequently need to visit a doctor, and it will be very expensive for both you and the hospital (2). Luckily, scientists, like Dr. Harald Ott, are finding innovative ways to solve this issue. By creating an entire new transplantable organ from scratch, patients can be healthy again without needing to wait for a donor.
Dr. Ott started his career in Austria, where he was a cardiac surgery resident. There, he realized the reality that patients with end-stage heart failure needed to either fix their heart or get a new one. He came to the University of Minnesota to do research on cell therapy, a technology which injects healthy cells into the injured tissue to repair it (3). However, Dr. Ott ran into an issue with this method. “The problem with cell therapy was really that people were trying to inject cardiomyocytes [heart muscle cells] into the heart—me included—and were then surprised that you didn’t form functional myocardium [heart muscle]. And if you think about it, it’s a little bit like throwing bricks on a pile and then looking surprised that a house didn’t form by itself,” Dr. Ott said. He had to change his approach to make sure the cells could actually form working tissue.
In order for the cells to create tissues effectively, they needed a scaffolding to build around. While there were different methods of achieving this effect, like creating the scaffolding from scratch, Dr. Ott used a novel approach. “When I was back home, I utilized a technique to wash cells out of the heart by digesting the heart with enzymes… so I said, can we turn this process upside down and refine a method to isolate a scaffold by removing all of the cells?” he thought. “That’s how I developed perfusion decellularization, which was basically a method to perfuse organs with soap, literally, detergents. And the right detergent combination yielded a process that would enable you to isolate the extracellular matrix scaffold.” The scaffolds would then be populated with healthy cells. Dr. Ott succeeded in creating beating hearts using this method. He then decided to move to Massachusetts General Hospital, hoping to achieve the same accomplishment for other organs.
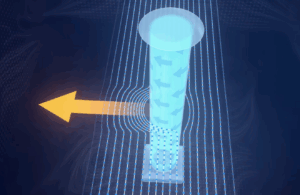
IVIVA’s Scaffold Printer (2)
One of the most prevalent challenges in trying to engineer a new organ was making sure it would be accepted in the body. Dr. Ott recalled a specific encounter where he was called out about this, admitting, “I stood at a conference and presented the heart data, and literally one of the surgeons got up and said, ‘You’re gonna make the worst heart in the world from cells that are gonna be embryonic, therefore rejected, and it’s complicated and total nonsense.” Ironically, Dr. Shinya Yamanaka was solving that exact issue at the same time—and actually won a Nobel Prize for it in 2012 (4). He discovered that cells in the body can be converted to pluripotent stem cells (basic cells that can do many functions) and then be used as organ cells. This breakthrough was impactful because engineered organs could be created by a patient’s own cells and therefore be accepted more easily by the body. “In a couple years, everyone in the world was doing this,” Dr. Ott stated, reaffirming the impact of this discovery. He describes the process very simply: “If you are a cell you have all of the genetic information in your nucleus, but you only read very little of it…If you remove the genetic restrictions, the cells all turn into a clean slate.” The stem cells can then be specialized to be used in different organs.
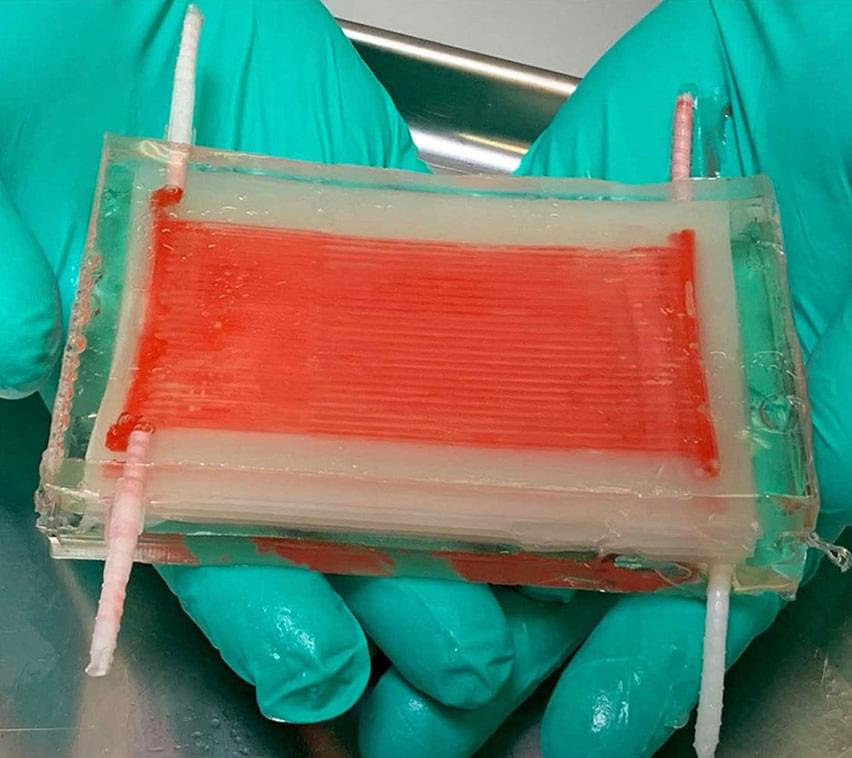
In 2013, Dr. Ott cofounded IVIVA Medical, which operates in Medford, MA, and strives to “generate autologous—meaning patient derived—renal grafts [kidneys] that get people off hemodialysis.” IVIVA creates their own kidney scaffolds instead of decellularized organs and implements Dr. Yamanaka’s pluripotent stem cell discovery to bring the scaffold to life (5). The issue with decellularized organs is that they are overcomplicated and can’t be customized. “It’s like saying, the homework assignment is not to build a bird, but to build a plane. If you put your surgeon hat on, you don’t really care if it’s a kidney, you care if it could get someone off dialysis,” Dr. Ott explained. They created a technology called thin film interposition to generate scaffolds that are simpler, but still fulfill their purpose. Starting with a membrane equivalent, IVIVA builds a perfusable structure around the membrane to allow for solute and gas exchange to achieve the core functions of a kidney.
IVIVA’s Kidney Scaffold (1)
The future’s looking bright for Dr. Ott and IVIVA. Older versions of their kidneys (without the induced pluripotent stem cells) have already succeeded on large animals, and now they just need to test again with the new technology. After a large animal test is successful, IVIVA can approach the FDA and test on humans. The regulatory path may not be as straightforward as it usually is, because it is difficult and unethical to do any tests other than testing on a sick patient. But with approval, soon the 1 in 4 people who will need a kidney transplant will be able to obtain one much quicker than waiting in years of waitlists (2).
Sources:
- The Kidney Transplant Waitlist. (2025, January 7). National Kidney Foundation. https://www.kidney.org/kidney-topics/kidney-transplant-waitlist
- on, L. (2022, April 7). Latest on Organ Engineering | Harald Ott | TEDxBoston. YouTube. https://www.youtube.com/watch?v=xCjG7igb0Yg
- Answers to your questions about stem cell research. (2024). Mayo Clinic. https://www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117
- The Nobel Prize in Physiology or Medicine 2012. (2024). NobelPrize.org. https://www.nobelprize.org/prizes/medicine/2012/yamanaka/facts/
- IVIVA Medical’s Journey in Kidney Engineering. (2024). IVIVA Medical. https://www.ivivamedical.com/kidney-tissue-engineering
Images:




Comments are closed.