The ethics and risks of editing embryonic DNA with CRISPR-Cas9 Technology
What would the world look like if we could pick the traits of our children? What if we could enhance intelligence, physical abilities, appearance, or prevent certain diseases before the child is even born? With Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) gene editing technology, scientists can accurately and precisely edit not only sections of DNA of already-born children or adults (somatic editing) but also the entire genetic makeup of human embryos (germline editing) (1). For years, the ethics and safety of gene editing have been widely debated in the scientific community (2). While furthering the development of CRISPR will almost certainly benefit society, there are questions about usage, accessibility, and potential unintended consequences that need to be answered before further significant action.
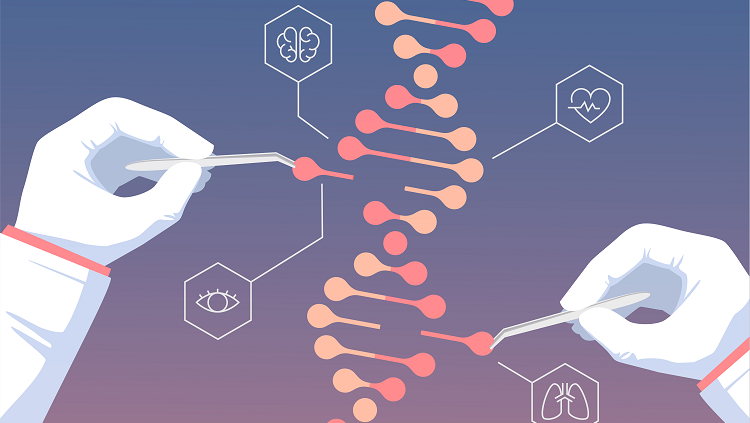
Image depicting the editing of DNA, and the ability to choose traits such as eye color, intelligence, and different health conditions. Shows the power of scientists, and the ease of the experiment.
As gene editing continues to advance, one topic of consideration is where to draw the ethical line in the usage of CRISPR on human embryos—including if it should be used at all (2). Humanity needs to establish if we have the right to modify our race in this way, and a pressing concern of reverting to eugenicist ideas and people unnaturally “playing God” needs to be addressed (1). In addition, the nature of germline editing (which affects all cells including the sperm and egg cells) creates permanent changes in DNA, affecting all subsequent generations (1). Modifying the appearance, intelligence, talent, and health of embryos means that the “ideal” human can be created; this reality would no doubt aid in the development of technology and society, but to what end is this ethical? Using CRISPR as a medical tool to prevent or cure genetic diseases and disabilities could prove to be revolutionary, but using it to modify physical or mental traits would likely induce significant consequences. Additionally, the risk of CRISPR being militarized needs to be considered (1). Even if regulations are set, there’s no current system to ensure their universal enforcement. A few years ago Chinese scientist He Jiankui illegally used CRISPR to edit the genes of embryos to make them more resistant to HIV (2). While the experiment was successful, this incident highlighted the possibility of CRISPR being used against protocol. Gene editing could solve issues for so many families and improve the futures of countless people, but its use needs to be considered cautiously and thoroughly.
If CRISPR ends up becoming widely available to the public, there are also concerns about the solidification of social divisions created as a result of unequal accessibility. Considering the U.S.’s current medical care system, gene editing procedures would be expensive and primarily accessible to people of a higher class (1). This fact means that people in the upper classes would have healthier, or even smarter and more attractive children, which would likely further enhance social divisions (2). Additionally, in today’s society, higher social classes are predominantly composed of white individuals. Providing them with a tool to further solidify their family’s societal standing would only reinforce the oppressive and racist systems we are striving to resolve. Until there is a way to ensure equal accessibility, there is no way for the release of CRISPR technology to the public to not cause chaos, hatred, and division.
It’s also important to consider that there are still studies being conducted on CRISPR’s potential side effects, and the risks are yet to be completely understood and controlled. The two forms of risks that come with using CRISPR are on-target effects and off-target effects (3). On-target effects are when the changes in the target code of DNA cause unintended side effects (1). Off-target effects describe accidental changes that occur in a different part of the DNA than where it was planned to be targeted (1). Both of these outcomes are currently unpredictable, and while work is being done to reduce their possibility, there is still research and revisions that need to be conducted until it is ready for wide-scale usage (3). It is necessary for the side effects and risks to be completely understood, especially in germline editing, where any error that occurs would affect not only the patient but also every future generation that shares their DNA. As far as current research on CRISPR is concerned, the lack of concrete knowledge on the effects of editing certain parts of DNA is too significant for it to be used confidently on embryos (2). Until it’s possible to accurately weigh the risks and the benefits, CRISPR shouldn’t be used in germline editing.
Diagram showing the process of CRISPR and the steps that it takes to edit DNA. It shows the different components involved and simply explains the process with a visual so it’s easy to understand.
The potential of CRISPR is unmeasurable, and it’s already being used greatly in the medical field to investigate cures for diseases including cancers, cardiovascular diseases, liver diseases, and Sickle Cell Anemia (4). Many successful cases of somatic editing have been conducted (2). The speed at which discoveries with CRISPR are being made makes it difficult for ethicists to keep up, but it’s important to take the time to ensure that germline editing is used correctly and responsibly (1). To use it prematurely, before its safety can be definitively known, would be dangerous and reckless, and the consequences could be disastrous. It’s likely that when concerns have been resolved, some form of embryonic editing will be safe, common, and highly regarded, as is the case with countless technologies in history (1). But, until that can happen, we can only try to understand what germline editing is and what it would mean for our society.
Bibliography:
- Cannon, W. (2019, January 9). Perspectives on gene editing. Harvard Gazette. Retrieved from https://news.harvard.edu/gazette/story/2019/01/perspectives-on-gene-editing/
- Henderson, H. and Halpern, J. (2024, November 23). CRISPR & Ethics. Innovative Genomics Institute. Retrieved from https://innovativegenomics.org/crisprpedia/crispr-ethics/
- Mengstie, M., Azezew, M., Dejenie, T., Teshome, A., Admasu, Fitalew., Teklemariam, A., Mulu, A., Agidew, M., Adugna, D., Geremew, H., and Abebe, C. (2024, January 18). Recent Advancements in Reducing the Off-Target Effect of CRISPR-Cas9 Genome Editing. Biologics, Volume 18, 21–28. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10802171/
- Li, T., Yang, Y., Qi, H., Cui, W., Zhang, L., Fu, X., He, X., Liu, M., Li, P., and Yu, T. (2023, January 16). CRISPR/Cas9 Therapeutics: Progress and Prospects. Signal Transduction and Targeted Therapy, 8(1). Retrieved from https://www.nature.com/articles/s41392-023-01309-7
Images:
- Richardson, W. (2019, July 15). What Is CRISPR Currently Being Used For?. Brainfacts.org. Retrieved from https://www.brainfacts.org/in-the-lab/tools-and-techniques/2019/crispr-explained-071519
- Blyth, P. (2023, April 25). CRISPR – Engineering a better future through genetics. SCOR. Retrieved from: https://www.scor.com/en/article/news-uk/crispr-engineering-better-future-through-genetics






Comments are closed.