How SHERLOCK and DETECR Diagnostic Tools Are Transforming Disease Detection
Imagine a tool so precise it can locate a single molecule of genetic material in a sea of millions within just minutes. Originally discovered as a bacterial defense mechanism, CRISPR systems have revolutionized biology and are renowned for their ability to edit, manipulate, and detect genetic material. In CRISPR systems, there are two classes with many different types and corresponding Cas-endonuclease proteins such as Cas9, Cas12, and Cas13. With a famous Cas9 protein known for its DNA-editing capabilities, Cas13a and Cas12a have been utilized in molecular diagnostics within tools like SHERLOCK and DETECTR, two examples of various uses for CRISPR (1).
Unlike Cas9, Cas13a targets RNA instead of DNA. Programmed with a specific sequence of its CRISPR RNA, Cas13a not only cleaves target RNA but also has a “collateral effect,” cutting nearby RNA once it recognizes the target. While this phenomenon initially posed challenges for genome editing, its discovery marked a turning point in the development of diagnostic tools (2).
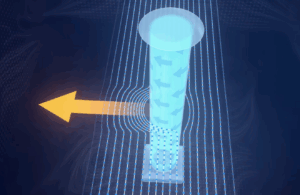
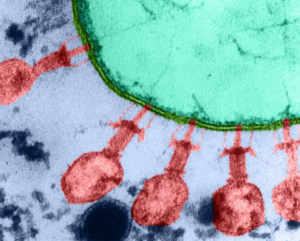
Detection with SHERLOCK and CRISPR protein Cas13a. https://www.nature.com/articles/s41596-019-0210-2
In science, challenges often inspire breakthroughs. Located in Cambridge, the Zhang lab utilized the non-specific activity of Cas13 to create SHERLOCK (Specific High-sensitivity Enzymatic Reporter unLOCKing), using CRISPR not to edit the genome, but rather to detect and diagnose biological material. After amplifying small amounts of DNA or RNA, SHERLOCK transforms low levels of target RNA into detectable signals. The Zhang Lab brought this innovation to real-world applications and applied SHERLOCK to diagnose the levels of Zika, a particularly difficult virus to diagnose due to its low amount of copies, in serums that return results in just a few hours, a game changer in time-critical diagnostics (3).
Building on this foundation, the second version of SHERLOCK made incredible advancements. This new version improved sensitivity by three and a half times more than Version 1 and, most importantly, added the ability to detect multiple targets simultaneously with orthogonal CRISPR enzymes. Additionally, with enhanced versatility, Version 2 can detect pathogens, viruses, and cancer-free mutations in cell-free DNA samples (4).
Similarly, DETECTR (DNA Endonuclease-Targeted CRIPSR Trans Reporter) is another powerful CRISPR-based diagnostic tool. The difference between SHERLOCK and DETECR is that DETECTR uses Cas12a’s collateral activity that cleaves nearby single-stranded DNA after target recognition (4). DETECR enables reporter molecules to emit a detectable signal when cleaved, allowing it to identify DNA-based pathogens with remarkable accuracy. This technology has even been applied to the detection COVID-19 (5).
Differences in SHERLOCK and DETECTOR. https://blog.addgene.org/finding-nucleic-acids-with-sherlock-and-detectr
SHERLOCK and DETECTR diagnostic tools have proven their ability to address global health challenges in detecting SARS-CoV-2 (COVID-19), Zika, and other diseases (3). These innovative tools have streamlined workflows and can be used with equipment as basic as a dipstick, offering simpler, faster alternatives to other diagnostic methods like PCR (4). From the unique properties of CRISPR enzymes, SHERLOCK and DETECTR are rapid, sensitive, and specific tools paving the way for crucial diagnostics––a critical factor in saving lives. CRISPR tools like SHERLOCK and DETECTR are just the tip of the iceberg in a transformative era of science and medicine. As researchers continue to make advances and uncover new possibilities, these innovations will lay the foundation for even more discoveries, offering hope for a solution to some of the most pressing challenges in global health and beyond.
Bibliography:
- Sinha, S., Adrian M Molina Vargas, Arantes, P., Patel, A., Mitchell R O’Connell, & Palermo, G. (2023). Unveiling the RNA-mediated allosteric activation discloses functional hotspots in CRISPR-Cas13a. Nucleic Acids Research, 52(2), 906–920. https://doi.org/10.1093/nar/gkad1127
- Adler, B. A., Hessler, T., Cress, B. F., Lahiri, A., Mutalik, V. K., Barrangou, R., … Doudna, J. A. (2022). Broad-spectrum CRISPR-Cas13a enables efficient phage genome editing. Nature Microbiology, 7(12), 1967–1979. https://doi.org/10.1038/s41564-022-01258-x
- Sherlock: Detecting disease with CRISPR. (2017, April 13). Retrieved January 23, 2025, from @broadinstitute website: https://www.broadinstitute.org/videos/sherlock-detecting-disease-crispr
- Mustafa MI, Makhawi AM.2021.SHERLOCK and DETECTR: CRISPR-Cas Systems as Potential Rapid Diagnostic Tools for Emerging Infectious Diseases. J Clin Microbiol59:10.1128/jcm.00745-20.https://doi.org/10.1128/jcm.00745-20
- Broughton, J. P., Deng, X., Yu, G., Fasching, C. L., Servellita, V., Singh, J., … Chiu, C. Y. (2020). CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology, 38(7), 870–874. https://doi.org/10.1038/s41587-020-0513-4







Comments are closed.